Migraine treatment innovations still have ways to go
Stephanie M. Lee
Published 3:02 pm, Saturday, July 19, 2014
Dr. Robert Cowan, head of the Stanford Headache Clinic helps migraine patient Nancy Mikhael, who is getting three rounds of Botox injections as part of her treatment. Photo: Leah Millis, The Chronicle
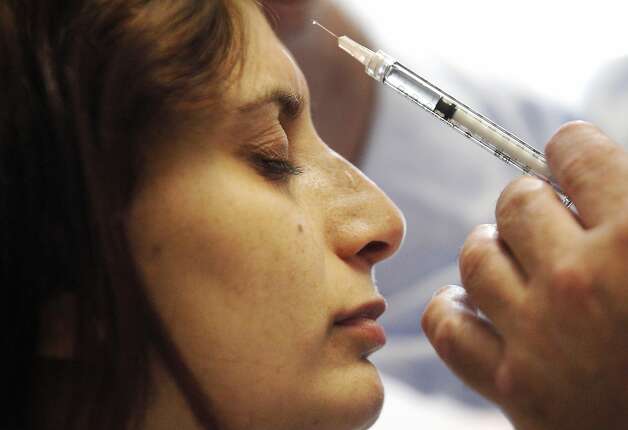
Dr. Robert Cowan, head of the Stanford Headache Clinic, administers a Botox injection to migraine patient Nancy Mikhael, 31, of San Jose. Photo: Leah Millis, The Chronicle
Few sensations are as grating as a head-splitting, nausea-inducing migraine that just won't disappear.
When aspirin and Advil aren't strong enough, patients often turn to the class of drugs called triptans, the prescription medicine DHE or even opioids. But these treatments do not work well in everyone and may have dangerous side effects.
So biotechnology companies, including one in San Mateo, are developing alternatives in an attempt to treat some of the 36 million Americans who suffer migraines. Also on the market are two head devices that use magnets or electricity, one of them developed at facilities in Sunnyvale.
The migraine market in developed countries will grow to nearly $5.4 billion in 2022, predicts the Decision Resources Group. "The unmet need is pretty strong," said Bethany Kiernan, a senior director for the health care market analysis firm. "Having a therapy that can either work to a greater extent in a larger percentage of the population, or that can better serve those patients that are currently underserved, has a real advantage in this market."
Universal relief may be a long way off, however. This month, a panel of California clinicians voiced support for Botox, the wrinkle-smoothing shots that are also approved to treat chronic migraines, defined as severe headaches at least 15 times per month.
At the same time, the doctors were skeptical about the two new head devices.
"These are all promising things, but we still know so little about what's actually going on," said Dr. Robert Cowan, director of Stanford University's Headache Clinic. "How come two different people get hit in the head the same way, one of them gets a lump in the head that goes away in few days or a week, and the other gets chronic headaches that never go away for the rest of their life?"
Seeking cause
Scientists are still trying to understand the exact causes of migraines. Labrys Biologics in San Mateo has been developing a drug that tries to prevent chronic and episodic headaches by binding a protein to a neuropeptide thought to play a role in migraines. In June, Israeli drug giant Teva Pharmaceutical Industries said it would acquire the company for up to $825 million.
But the field has seen some high-profile flops. Last year, Allergan spent nearly $1 billion to acquire a new, inhalable migraine drug, only for the Food and Drug Administration to reject it out of safety concerns.
Allergan also owns Botox. In 2010, the cosmetic injections won approval to treat adults with chronic migraines. Current therapy recommendations call for injections every three months.
Compared to no treatment at all, Botox seems to have small benefits in some patients, the California Technology Assessment Forum agreed this month. The panel of physicians reviews medical issues three times a year.
The group was not as convinced of the merits of SpringTMS, a handheld device that delivers a brief pulse of energy, or transcranial magnetic stimulation, to the back of the head. It is supposed to generate magnetic fields that interrupt abnormal electrical activity associated with migraines.
The device won federal approval in May and is made by eNeura Therapeutics, whose headquarters are in Baltimore and whose research and development facilities are in Sunnyvale. CEO David Rosen said the product may appeal to the estimated 10 to 15 percent of migraine patients who are not eligible for drugs or other treatments.
"It would make sense for a specialist to consider use of the device and prescribe use of the device for patients who don't have any other treatment option," he said. "What are you going to do for these people besides say, 'I don't have a treatment option for you?' "
But the panel decided there was inconclusive evidence to demonstrate the SpringTMS was better than standard treatments. Patients in one study reported some relief after using the device, but other measures, such as whether patients became less sensitive to light and sound, did not significantly differ from the placebo users.
"There really was no difference between the device and a sham stimulator," said Dr. Rita Redberg, a cardiologist at UCSF, who sided with the majority of the panel. It was "just putting something on your head and thinking you were being treated for migraines."
The panel was similarly skeptical of Cefaly, a $449 headband designed to deliver electrical impulses to nerves that transmit migraine pain. Approved in March, it is supposed to be worn for 20 minutes each day to reduce the frequency of migraines - but the panel found the supporting evidence inconclusive.
Few options
At the same time, the group acknowledged that many patients lack viable options. The popular migraine drugs known as triptans, for example, temporarily narrow blood vessels, an effect that makes them off-limits for people with coronary artery disease and other vascular conditions.
Frustrated patients often seek out opioids in the emergency room, but opioids can be dangerous. In a year, the panel estimated, 20,000 patients in California develop chronic migraines because of opioid overuse, and 3,000 become addicted. So the demand for safe and effective alternatives is urgent.
"Headache care is 50 years behind things like diabetes and cancer," said Cowan, the Stanford doctor, who was an adviser to the panel. "It just hasn't had the attention, hasn't had the funding, in order to get to the answers we need."
Stephanie M. Lee is a San Francisco Chronicle staff writer. E-mail: slee@sfchronicle.comTwitter: @stephaniemlee
No comments:
Post a Comment